The most important function of the intestinal barrier is to balance the absorption of nutrients, electrolytes and water from the lumen into the blood flow and to prevent the entry of pathogenic microorganisms and toxic luminal substances. Additional regulation of this exchange of molecules between the environment and the host over the intestinal barrier affects the balance between immunity and tolerance to both self and non-self-antigens.
Table of Contents
What’s the significance of intestinal barrier function?
From a structural standpoint, intestinal barrier function is maintained by many components like a mucous coating and a single layer of epithelial cells connected with tight junctions. The mucus layer is made up of secretory immunoglobulin (Ig) A and antimicrobial peptides which cover the epithelial cell lining to ease gastrointestinal transport, or GI transport, and provide a protective coating against bacterial invasion. The colonic mucous coating consists of two layers, an outer and inner layer consisting of gel forming highly glycosylated proteins known as mucins. These are generated and preserved by goblet cells which repair the internal mucus layer roughly every hour. These dynamic procedures are subject to extensive and constant interactions between the gut microbiota, where the disruption of such may have consequences for the sustenance of crucial intestinal barrier function.
Tight junctions are complex protein structures which contain transmembrane proteins like claudin, occludin, and tricullulin. These transmembrane proteins connect with all the opposing plasma membrane, forming a mechanical network between epithelial cells and establishing a barrier for the paracellular diffusion of fluid and solutes. The structure of the intestinal barrier is shaped by the end of the first trimester. Epithelial cells with microvilli, goblet and enteroendocrine cells, develop by week eight of gestation and tight junctions can be observed from week ten. Functional development of the intestinal barrier continues from the post natal period and can be affected by both feeding diet and mode. By way of instance, as demonstrated to be exemplified by the underdeveloped intestinal barrier of a premature baby, disruptions in the development process of the intestinal barrier can predispose the infant to immune disorders.
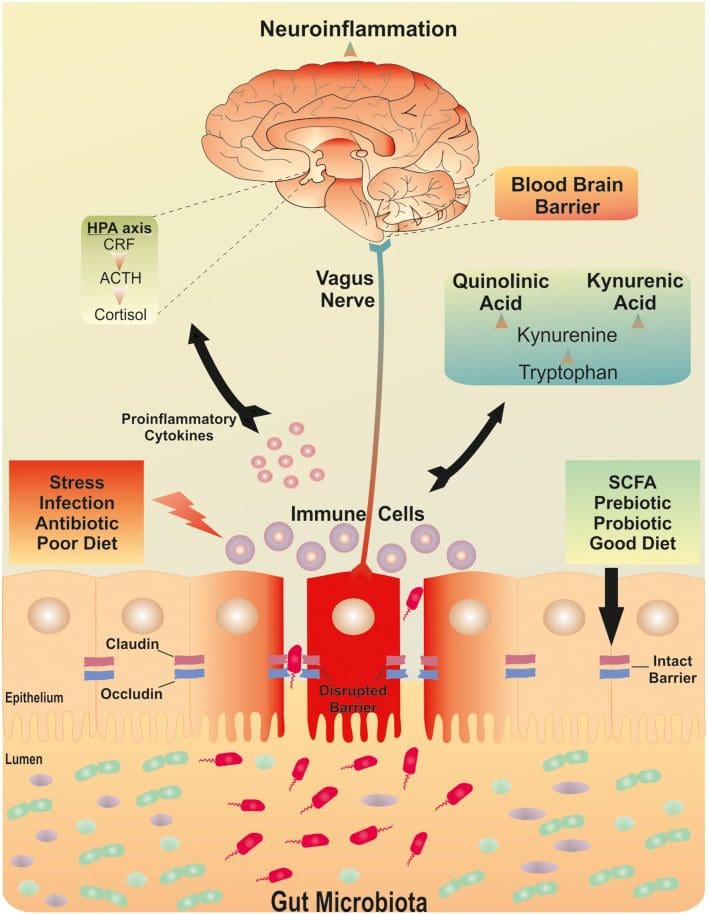
The gut microbiota, from the first days of life, is unstable and not especially varied in its own make-up. By age three, however, the microbiota composition resembles the adult-like profile. Many different factors might also influence the course of microbiota growth, such as gestational age, mode of delivery, type of feeding, antibiotic usage, and exposure to family members and pets. The intestinal barrier functions as a shield that may be altered by the gut microbiota or its metabolites. The mechanisms underlying the balance of the epithelial barrier can be complicated. Recent evidence also indicates a novel role for non-coding RNAs, like microRNAs, as important intermediaries for the connections between host epithelial cells, immune cells and the gut microbiota. Alterations to the gut microbiota are correlated with concomitant gut barrier dysfunction in both intestinal and extra-intestinal diseases. The role of the gut microbiota in disrupting the intestinal barrier in stress-related neuropsychiatric conditions, such as depression, hasn’t been completely researched.
Brain-Gut Microbiota Axis
Interactions between the gut and the brain are controlled at nerve, endocrine, and immune levels of the human body. These pathways are within the effect of the gut microbiota and collectively, they include the brain-gut-microbiota axis. An essential purpose of the gut microbiota is the development and balace of the intestinal barrier throughout an individual’s lifespan. It’s possible that subtle alterations within microbiota expenditure or care in early life might serve a vulnerability factor, affecting (neuro)endocrine and (neuro)immune signaling pathways of the brain-gut-microbiota axis, which can then predispose individuals to stress-related diseases during adulthood. Animals devoid of gut microbiota show reduced levels of anxiety but an exaggerated neuroendocrine response to stress. The most evident impact created by the microbiota might happen early in life through crucial neurodevelopmental phases. It’s clear that the gut microbiota is needed for the normal development of the hypothalamic pituitary adrenal, or HPA, axis and there appears to be a definite period in early life when colonization should occur to ensure normal development of the crucial stress signaling pathway. A theory suggests that restricting less microbial biodiversity can lead to the growth in chronic inflammatory diseases, like some subtypes of depression.
The interaction between the immune system, the gut microbiota and the intestinal barrier might be of specific value to health in the opposite extreme of aging. Aging is characterized by long-term inflammation, termed “inflammaging”, according to elevated circulating levels of Tumor necrosis factor-alpha, or TNF-α, interleukin-6, or IL-6 and C-reactive protein, CRP; inflammatory molecules known to influence mood and cognition. Due to the simple fact that the gut microbiota are crucial regulators of immune function and inflammatory reactions, it’s possible that an alteration in the composition of the gut microbiota during aging plays a part in the slow activation of their immune system and inflammaging, potentially through an effect on intestinal permeability. It’s been demonstrated that the elderly have a different microbiota profile, characterized by larger inter-individual variations in comparison to younger adults. Needless to say, differences in microbiota composition were more pronounced between frail elderly subjects and healthy elderly subjects. Additionally, certain gut microbiota signatures have been connected to measures of frailty, co-morbidity, nutrient status, and markers of inflammation.
Intestinal Barrier Function and Stress
Stress can affect the developmental progress of the intestinal barrier, which has also been associated with an increase in intestinal permeability. The consequences of stress on intestinal permeability are complicated and are believed to involve the gut and the brain. Corticotrophin releasing factor, or CRF, and its receptors (CRFR1 and CRFR2), play an integral role in stress-induced intestinal permeability dysfunction. In reaction to an acute stressor, colonic paracellular permeability increases and may be connected to the growth of visceral hypersensitivity. At a mouse model of chronic depression, elevated central CRH expression occurred concomitantly with alterations from the gut microbiota.
Early life stress has also been proven to boost plasma corticosterone in rat pups and is connected with an increase in intestinal permeability and bacterial translocation into the liver and spleen. This effect seemed to predominate in the colon. Naturally, stress-induced changes in the HPA axis and autonomic nervous system screen sensitivity to probiotic intervention (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175). Furthermore, these probiotics additionally revived colonic tight junction integrity in stressed mice. Probiotics have further been proven to affect bacterial adhesion and translocation to mesenteric lymph nodes in reaction to stress. Lactobacillus farciminis, by way of instance, not merely inhibits stress-induced changes in permeability, HPA axis activity, endotoxaemia, and neuroinflammation, but also beneficially affects the mucus barrier. Human studies also confirmed that acute-stress paradigms can influence intestinal permeability. During a public talking based stressor, little intestinal permeability was significantly improved, however, this was only observed in subjects who also reacted with a substantial elevation of cortisol. In a contrasting acute stress model utilizing a cold pain stressor, albumin permeability increased, though only in females.
Stressful early-life situations are strongly connected to the development of depression later in life. The interaction between stress, the HPA axis and the immune system is well recognized. In the past few years it has been discovered that the gut microbiota mediates this interaction. Early-life maternal separation, by way of instance, leads to a significant reduction in the amount of fecal Lactobacillus three days post-separation, associated with stress-related behaviors. At a mouse model of societal disturbance, stress-induced alterations in the microbiota were accompanied by changes in cytokine and chemokine levels. Many different research studies have confirmed that stress can change gut microbiota composition. This is also related prenatally, as babies of mothers with elevated self-reported stress and high salivary cortisol levels throughout pregnancy had a higher relative amount of Proteobacteria and lower relative amounts of lactic acid bacteria (Lactobacillus, Lactoccus, Aerococcus) and Bifidobacteria. But, it’s unclear if this effect was mediated through esophageal parasitic transmission or via microbiota-independent situations. Nonetheless, those babies with altered microbiota composition exhibited a greater degree of GI symptoms and allergies, highlighting the practical effects of abnormal colonization patterns in early life. Recent preclinical evidence demonstrated that maternal stress altered the vaginal microbiota, diminishing Lactobacillus, with consequences on its metabolic profile and neurodevelopment in the offspring.
A dysfunctional intestinal barrier may allow a microbiota driven proinflammatory condition with consequences for the brain, as seen on Figure ​1. The progress of the course of action isn’t yet very clear. An increase in intestinal permeability could predict mucosal inflammation to cause the inflammatory reaction and end up at a feed-forward cycle involving inflammatory reactions and intestinal barrier dysfunction. This may then maintain and exacerbate the very low grade inflammatory reaction. Alternately, systemic inflammation can improve intestinal barrier permeability and allow translocation of commensal bacteria with additional consequences for systemic inflammation. The origin of the very low grade inflammation that has been reported in depression hasn’t yet been isolated to a certain source.
Greater IgA- and – IgM-mediated immune reactions directed against lipopolysaccharides, or LPS, of specific commensal g negative gut bacteria have been demonstrated in depressed patients. The implication being that the existence of these reactions may have occurred following the disturbance of the intestinal barrier. Additionally, bacterial DNA was discovered in whole serum from patients with depression that also demonstrated increased Toll-like receptor (TLR)-4 expression on peripheral mononuclear blood cells when compared with healthy controls.
Two printed cross sectional studies have researched the gut microbiota composition in depression. The first discovered that an increase in Bacteroidales along with a reduction in Lachnospiraceae compared to controls. But, there have been no major differences in species richness, α-diversity, or operational taxonomic units. In the next study, increased amounts of Enterobacteriaceae and Alistipes were detected. Moreover, Fecalibacterium amounts decreased in the depressed group and negatively correlated with severity of depressive symptoms. From the context of alcohol abuse, a connection between the gut microbiota, intestinal barrier function and comorbid depression has just been reported. Microbiota-derived LPS and peptidoglycans, or PGN, were proven to cross the intestinal barrier and trigger their various receptors, including TLR4 and TLR2 in peripheral blood mononuclear cells. Although chronic alcohol ingestion inhibited the NF-κB pathway, it triggered protein kinase/activator protein pathway and IL-8 and IL-1B. By comparison, short-term alcohol withdrawal has been connected to the retrieval of TLR4 receptors. The identical group also revealed that increased intestinal permeability happened in a sub group of alcohol-dependent subjects that were associated with greater depression and anxiety scores in addition to a modified gut microbiota profile.
Information referenced from the National Center for Biotechnology Information (NCBI) and the National University of Health Sciences. The scope of our information is limited to chiropractic and spinal injuries and conditions. To discuss the subject matter, please feel free to ask Dr. Jimenez or contact us at 915-850-0900 .
By Dr. Alex Jimenez

Additional Topics: Wellness
Overall health and wellness are essential towards maintaining the proper mental and physical balance in the body. From eating a balanced nutrition as well as exercising and participating in physical activities, to sleeping a healthy amount of time on a regular basis, following the best health and wellness tips can ultimately help maintain overall well-being. Eating plenty of fruits and vegetables can go a long way towards helping people become healthy.

ADDITIONAL TOPIC: EXTRA EXTRA: Treating Back Pain
Post Disclaimer
Professional Scope of Practice *
The information herein on "Stress, the Gut Microbiota & Intestinal Barrier Function" is not intended to replace a one-on-one relationship with a qualified health care professional or licensed physician and is not medical advice. We encourage you to make healthcare decisions based on your research and partnership with a qualified healthcare professional.
Blog Information & Scope Discussions
Welcome to El Paso's Premier Wellness, Personal Injury Care Clinic & Wellness Blog, where Dr. Alex Jimenez, DC, FNP-C, a Multi-State board-certified Family Practice Nurse Practitioner (FNP-BC) and Chiropractor (DC), presents insights on how our multidisciplinary team is dedicated to holistic healing and personalized care. Our practice aligns with evidence-based treatment protocols inspired by integrative medicine principles, similar to those on this site and our family practice-based chiromed.com site, and focuses on restoring health naturally for patients of all ages.
Our areas of multidisciplinary practice include Wellness & Nutrition, Chronic Pain, Personal Injury, Auto Accident Care, Work Injuries, Back Injury, Low Back Pain, Neck Pain, Migraine Headaches, Sports Injuries, Severe Sciatica, Scoliosis, Complex Herniated Discs, Fibromyalgia, Chronic Pain, Complex Injuries, Stress Management, Functional Medicine Treatments, and in-scope care protocols.
Our information scope is multidisciplinary, focusing on musculoskeletal and physical medicine, wellness, contributing etiological viscerosomatic disturbances within clinical presentations, associated somato-visceral reflex clinical dynamics, subluxation complexes, sensitive health issues, and functional medicine articles, topics, and discussions.
We provide and present clinical collaboration with specialists from various disciplines. Each specialist is governed by their professional scope of practice and their jurisdiction of licensure. We use functional health & wellness protocols to treat and support care for musculoskeletal injuries or disorders.
Our videos, posts, topics, and insights address clinical matters and issues that are directly or indirectly related to our clinical scope of practice.
Our office has made a reasonable effort to provide supportive citations and has identified relevant research studies that support our posts. We provide copies of supporting research studies upon request to regulatory boards and the public.
We understand that we cover matters that require an additional explanation of how they may assist in a particular care plan or treatment protocol; therefore, to discuss the subject matter above further, please feel free to ask Dr. Alex Jimenez, DC, APRN, FNP-BC, or contact us at 915-850-0900.
We are here to help you and your family.
Blessings
Dr. Alex Jimenez DC, MSACP, APRN, FNP-BC*, CCST, IFMCP, CFMP, ATN
email: [email protected]
Multidisciplinary Licensing & Board Certifications:
Licensed as a Doctor of Chiropractic (DC) in Texas & New Mexico*
Texas DC License #: TX5807, Verified: TX5807
New Mexico DC License #: NM-DC2182, Verified: NM-DC2182
Multi-State Advanced Practice Registered Nurse (APRN*) in Texas & Multi-States
Multi-state Compact APRN License by Endorsement (42 States)
Texas APRN License #: 1191402, Verified: 1191402 *
Florida APRN License #: 11043890, Verified: APRN11043890 *
Colorado License #: C-APN.0105610-C-NP, Verified: C-APN.0105610-C-NP
New York License #: N25929, Verified N25929
License Verification Link: Nursys License Verifier
* Prescriptive Authority Authorized
ANCC FNP-BC: Board Certified Nurse Practitioner*
Compact Status: Multi-State License: Authorized to Practice in 40 States*
Graduate with Honors: ICHS: MSN-FNP (Family Nurse Practitioner Program)
Degree Granted. Master's in Family Practice MSN Diploma (Cum Laude)
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card
Licenses and Board Certifications:
DC: Doctor of Chiropractic
APRNP: Advanced Practice Registered Nurse
FNP-BC: Family Practice Specialization (Multi-State Board Certified)
RN: Registered Nurse (Multi-State Compact License)
CFMP: Certified Functional Medicine Provider
MSN-FNP: Master of Science in Family Practice Medicine
MSACP: Master of Science in Advanced Clinical Practice
IFMCP: Institute of Functional Medicine
CCST: Certified Chiropractic Spinal Trauma
ATN: Advanced Translational Neutrogenomics
Memberships & Associations:
TCA: Texas Chiropractic Association: Member ID: 104311
AANP: American Association of Nurse Practitioners: Member ID: 2198960
ANA: American Nurse Association: Member ID: 06458222 (District TX01)
TNA: Texas Nurse Association: Member ID: 06458222
NPI: 1205907805
| Primary Taxonomy | Selected Taxonomy | State | License Number |
|---|---|---|---|
| No | 111N00000X - Chiropractor | NM | DC2182 |
| Yes | 111N00000X - Chiropractor | TX | DC5807 |
| Yes | 363LF0000X - Nurse Practitioner - Family | TX | 1191402 |
| Yes | 363LF0000X - Nurse Practitioner - Family | FL | 11043890 |
| Yes | 363LF0000X - Nurse Practitioner - Family | CO | C-APN.0105610-C-NP |
| Yes | 363LF0000X - Nurse Practitioner - Family | NY | N25929 |
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card




 Again, We Welcome You.
Again, We Welcome You.
Comments are closed.