Autoimmune diseases are characterized by tissue damage and loss of function caused when an immune reaction affects specific organs of the human body. The following article is focused on celiac disease, or CD, an autoimmune enteropathy, and type 1 diabetes, or T1D, a hyperglycosaemia resulting from an autoimmune response which targets the insulin-producing pancreatic islet cells.
Table of Contents
What causes autoimmune diseases and intestinal barrier dysfunction?
Although environmental and genetic factors are strongly believed to be involved in the pathogenesis of autoimmunity, there’s little understanding about the causing agent or genetic makeup underlying the disease. There’s growing evidence that intestinal barrier dysfunction as well as increased intestinal permeability plays a vital function in the development of various autoimmune disorders, such as CD and type 1 diabetes. Thus, researchers discovered that apart from environmental and genetic factors, a decrease in intestinal barrier function is necessary to develop autoimmune diseases. Each one of those factors will be briefly discussed in the following articles.
Genetic Factors of Autoimmune Diseases
Celiac Disease
The intricate connection between environmental and genetic factors resulting in the deficiency, or reduction of gluten tolerance remains poorly known. Prevalent research studies conducted in Europe and current statistics demonstrated in the U.S.A. both revealed a greater incidence of CD among asymptomatic first-degree relatives of celiac disease patients than among the overall population. Of 24 reported pairs of identical twins with CD, about 75 percent were concordant for disease. However, it seems that there are instances of discordance for CD among monozygotic twins. When it’s assumed that twins consume comparable dietary grains, for example, this discordance indicates the significance of environmental factors in disease expression. Course II HLA genotypes DQ2 and DQ8 play a significant predisposing function, being found in nearly 100 percent of individuals. HLA class II genes are highly polymorphic, with the vast majority of polymorphisms encoding the domain of this molecule which constitute the sides and bottom of the peptide binding groove. Various mixtures of HLA-DQ haplotypes influence the risk of disease, which is a lot greater in regions demonstrating a dual copy of the DQB * 02 gene (DQ2/DQ2 and DQ2/DR7) compared to the DQ8 (DR4)/X genotype. Inheritance of particular HLA-DQ genotypes revealed 40 percent of their genetic predisposition to celiac disease, whereas the remaining 60 percent is connected to an intricate, still-undefined mosaic of non-HLA genes, each of which provides a small contribution to the chance of CD growth.
By way of instance, recent research studies have clarified the non-HLA genes encoding for proteins involved in the regulation of intestinal permeability to be related to susceptibility for celiac disease. Polymorphisms in the MYO9B receptor have been demonstrated to be associated with greater susceptibility for gluten-sensitive enteropathy, or GSE, in GSE patients, and lately, the TJ-related genes PARD3 and MAGI2 were also proven to be associated with CD.
Type 1 Diabetes
The initial type 1 diabetes susceptibility genes to be identified by researchers were the HLA genes, based on chromosome 6p21. Subsequent research studies revealed a connection between the disease and the insulin gene region on chromosome 11p. From the mid-to-late 1990s, higher throughput screening of the whole human genome in families with 2 or more affected siblings was utilized to characterize other chromosomal domains that may contain susceptibility genes for T1D. More than 20 loci revealed evidence for its connection with the disorder in various data collections. Each of the research studies always demonstrated an association to the HLA gene region (designated IDDM1). Several genome displays, in conjunction with family-based evidence, also supported a role for the insulin receptor area (designated IDDM2) in disease susceptibility. Linkage to eight extra loci was duplicated in separate data sets: IDDM4 (chromosome 11q13), IDDM5 (chromosome 6q25), IDDM7 (chromosome 2q31), IDDM8 (chromosome 6q27), IDDM10 (chromosome 10p11-q11), IDDM12 (chromosome 2q33), IDDM13 (chromosome 2q35), along with IDDM15 (chromosome 6q21). Additionally, the locus-designated IDDM6 (chromosome 18q21) revealed consistent evidence for association with the disorder from family data. Even though the chromosomal locations of those loci are well known, the exact identity of these susceptibility genes in those areas remains to be confirmed. The genome displays confirmed, as for CD, that the HLA gene region (IDDM1 locus) is the significant genetic determinant of the risk of disease, accounting for 42 percent of the familial inheritance of T1D. The IDDM2 locus (the insulin receptor region) lead to an extra 10 percent of hereditary susceptibility.
In addition, polymorphisms from the MYO9B receptor have been demonstrated to be related to susceptibility for type 1 diabetes. This observation indicates that the susceptibility for the growth of the CD and T1D is connected to genes associated in intestinal permeability. The section describing the factor of intestinal barrier function in the development of autoimmune diseases like celiac disease and type 1 diabetes will be discussed below.
Intestinal Barrier Factors of Autoimmune Diseases
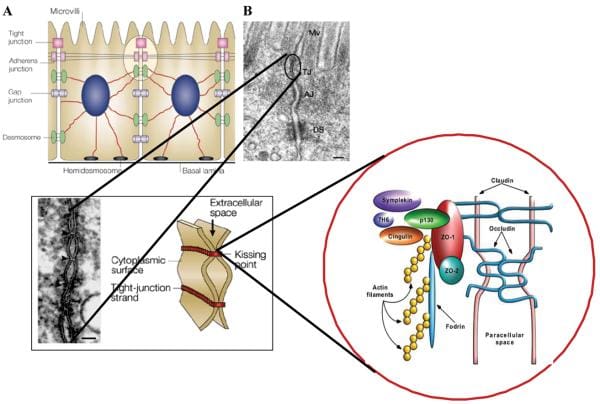
The Zonulin System
In the last few decades, much has been found about the structure, function and regulation of TJ, as seen on Figure 1. However, the exact mechanism(s) where they function are not entirely understood. The discovery of zonula occludens toxin, or Zot, an enterotoxin elaborated by Vibrio cholerae which reversibly unlocks the TJ, improved the knowledge of the complex mechanisms which control the intestinal epithelial paracellular pathway. Zot activity is mediated via an overflow of intracellular events which contribute to the PKCα-dependent polymerization of actin microfilaments which have been found to regulate the paracellular pathway. By employing immunofluorescence binding studies, researchers discovered that Zot binding changes within the gut, and are detectable from the jejunum and distal ileum, but not in the colon, while it also decreases over the villous-crypt axis. This binding supply coincides with the adrenal epithelial paracellular permeability across the villous axis45 and together with the regional impact of Zot on intestinal permeability and on F-actin redistribution from the cells of the villi. The combined data demonstrated that zonula occludens toxin regulates the tight junction in a quick, reversible, and reproducible manner. According to this monitoring, it was hypothesized that Zot could mimic the impact of a functionally and immunologically related, physiologically relevant endogenous modulator of epithelial TJs. V. cholerae-derived Zot and individual zonulin both behave on intestinal TJs, as seen on Figure 2, and exhibit the identical regional action coincident with Zot receptor distribution from the gut, suggesting that both of these molecules interact with the exact same receptor. Researchers also contrasted the main amino acid structures of Zot and zonulin to offer insights into the structural requirements for ligand involvement of the receptor coupled to autoimmune TJ regulation. The NH2-termini of zonulin and also the Zot active fragment demonstrated a frequent octapeptide theme (GGVLVQPG) which is essential for intestinal receptor binding. The synthetic octapeptide motif, called AT1001, has since been proven to be an efficient inhibitor of the two Zot and zonulin. The physiological function(s) of this zonulin system still needs to be concluded. This pathway seems to be involved in many functions, such as TJ regulation accountable for the motion of fluid, macromolecules and leukocytes between the blood and the intestinal lumen and vice versa. Another potential physiological function of intestinal zonulin is the defense from microorganism colonization of the proximal intestine, known as innate immunity.
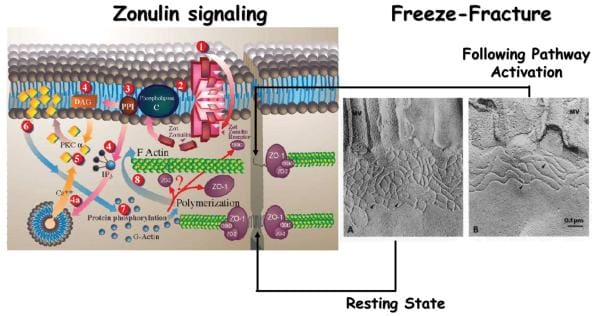
Zonulin and Autoimmune Diseases
Intestinal TJ dysfunction can result through many different clinical conditions, such as food allergies, and infections of the gastrointestinal tract, autoimmune diseases, and inflammatory bowel diseases. Several autoimmune diseases are characterized by intestinal barrier dysfunction. To learn more about the possibility that zonulin is included in the pathogenesis of the disease, researchers concentrated their studies on CD and T1D, two autoimmune diseases where the finely tuned regulation of intestinal TJ permeability has been missing. To be sure if zonulin creation is an issue through the acute phase of celiac disease, intestinal cells from patients with active CD and non-CD controls were probed for zonulin expression. Quantitative immunoblotting of adrenal gland lysates from active CD patients confirmed the growth of zonulin protein in comparison with control cells. The zonulin upregulation during the acute phase of CD was confirmed by quantifying zonulin immersion in sera of all 189 CD patients with a sandwich ELISA. In contrast to healthy controls, celiac disease subjects demonstrated considerably higher zonulin serum levels (P < 0.000001) during the acute stage of the disease which decreased following a gluten free diet. Similar results had been obtained in T1D subjects. From the BBDP rat version of T1D, research studies reported that zonulin-dependent increases in intestinal permeability precede the onset of T1D by 2–3 months. Oral administration of this zonulin inhibitor AT1001 into BBDP rats blocked autoantibody formation and zonulin-mediated intestinal permeability growth, reducing the prevalence of type 1 diabetes. Additionally, feeding BBDP rats a gluten free diet decreased the serum zonulin levels, as seen on Figure 3. Taken collectively, these research studies demonstrated that the zonulin-dependent reduction of intestinal barrier function is among the first actions in the pathogenesis of T1D from the BBDP animal model of the disorder.
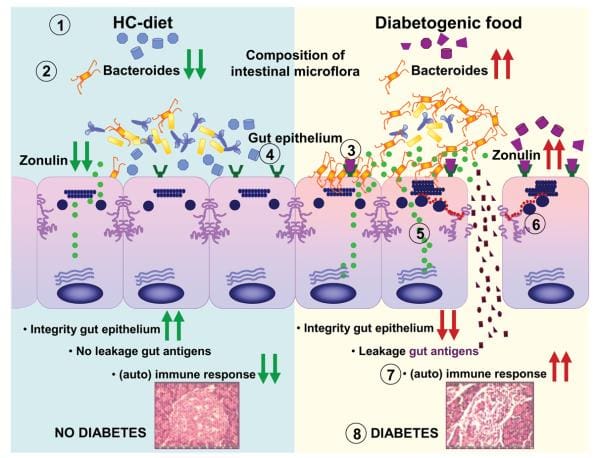
The participation of zonulin in T1D pathogenesis was authenticated by data and evidence in humans, demonstrating a sizable subgroup of type 1 diabetes patients has elevated hemoglobin zonulin levels that were associated with increased intestinal permeability and changes in claudin-1, claudin-2, and myosin IXB genes saying. Furthermore, research studies also provided preliminary evidence indicating that, like from the BBDP rat version of this disorder, zonulin upregulation precedes the identification of the disorder at T1D patients.
Information referenced from the National Center for Biotechnology Information (NCBI) and the National University of Health Sciences. The scope of our information is limited to chiropractic and spinal injuries and conditions. To discuss the subject matter, please feel free to ask Dr. Jimenez or contact us at 915-850-0900 .
By Dr. Alex Jimenez

Additional Topics: Wellness
Overall health and wellness are essential towards maintaining the proper mental and physical balance in the body. From eating a balanced nutrition as well as exercising and participating in physical activities, to sleeping a healthy amount of time on a regular basis, following the best health and wellness tips can ultimately help maintain overall well-being. Eating plenty of fruits and vegetables can go a long way towards helping people become healthy.

WELLNESS TOPIC: EXTRA EXTRA: Managing Workplace Stress
Post Disclaimer
Professional Scope of Practice *
The information herein on "Genetic Factors for Autoimmune Diseases & Intestinal Barrier Function" is not intended to replace a one-on-one relationship with a qualified health care professional or licensed physician and is not medical advice. We encourage you to make healthcare decisions based on your research and partnership with a qualified healthcare professional.
Blog Information & Scope Discussions
Welcome to El Paso's Premier Wellness, Personal Injury Care Clinic & Wellness Blog, where Dr. Alex Jimenez, DC, FNP-C, a Multi-State board-certified Family Practice Nurse Practitioner (FNP-BC) and Chiropractor (DC), presents insights on how our multidisciplinary team is dedicated to holistic healing and personalized care. Our practice aligns with evidence-based treatment protocols inspired by integrative medicine principles, similar to those on this site and our family practice-based chiromed.com site, and focuses on restoring health naturally for patients of all ages.
Our areas of multidisciplinary practice include Wellness & Nutrition, Chronic Pain, Personal Injury, Auto Accident Care, Work Injuries, Back Injury, Low Back Pain, Neck Pain, Migraine Headaches, Sports Injuries, Severe Sciatica, Scoliosis, Complex Herniated Discs, Fibromyalgia, Chronic Pain, Complex Injuries, Stress Management, Functional Medicine Treatments, and in-scope care protocols.
Our information scope is multidisciplinary, focusing on musculoskeletal and physical medicine, wellness, contributing etiological viscerosomatic disturbances within clinical presentations, associated somato-visceral reflex clinical dynamics, subluxation complexes, sensitive health issues, and functional medicine articles, topics, and discussions.
We provide and present clinical collaboration with specialists from various disciplines. Each specialist is governed by their professional scope of practice and their jurisdiction of licensure. We use functional health & wellness protocols to treat and support care for musculoskeletal injuries or disorders.
Our videos, posts, topics, and insights address clinical matters and issues that are directly or indirectly related to our clinical scope of practice.
Our office has made a reasonable effort to provide supportive citations and has identified relevant research studies that support our posts. We provide copies of supporting research studies upon request to regulatory boards and the public.
We understand that we cover matters that require an additional explanation of how they may assist in a particular care plan or treatment protocol; therefore, to discuss the subject matter above further, please feel free to ask Dr. Alex Jimenez, DC, APRN, FNP-BC, or contact us at 915-850-0900.
We are here to help you and your family.
Blessings
Dr. Alex Jimenez DC, MSACP, APRN, FNP-BC*, CCST, IFMCP, CFMP, ATN
email: [email protected]
Multidisciplinary Licensing & Board Certifications:
Licensed as a Doctor of Chiropractic (DC) in Texas & New Mexico*
Texas DC License #: TX5807, Verified: TX5807
New Mexico DC License #: NM-DC2182, Verified: NM-DC2182
Multi-State Advanced Practice Registered Nurse (APRN*) in Texas & Multi-States
Multi-state Compact APRN License by Endorsement (42 States)
Texas APRN License #: 1191402, Verified: 1191402 *
Florida APRN License #: 11043890, Verified: APRN11043890 *
Colorado License #: C-APN.0105610-C-NP, Verified: C-APN.0105610-C-NP
New York License #: N25929, Verified N25929
License Verification Link: Nursys License Verifier
* Prescriptive Authority Authorized
ANCC FNP-BC: Board Certified Nurse Practitioner*
Compact Status: Multi-State License: Authorized to Practice in 40 States*
Graduate with Honors: ICHS: MSN-FNP (Family Nurse Practitioner Program)
Degree Granted. Master's in Family Practice MSN Diploma (Cum Laude)
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card
Licenses and Board Certifications:
DC: Doctor of Chiropractic
APRNP: Advanced Practice Registered Nurse
FNP-BC: Family Practice Specialization (Multi-State Board Certified)
RN: Registered Nurse (Multi-State Compact License)
CFMP: Certified Functional Medicine Provider
MSN-FNP: Master of Science in Family Practice Medicine
MSACP: Master of Science in Advanced Clinical Practice
IFMCP: Institute of Functional Medicine
CCST: Certified Chiropractic Spinal Trauma
ATN: Advanced Translational Neutrogenomics
Memberships & Associations:
TCA: Texas Chiropractic Association: Member ID: 104311
AANP: American Association of Nurse Practitioners: Member ID: 2198960
ANA: American Nurse Association: Member ID: 06458222 (District TX01)
TNA: Texas Nurse Association: Member ID: 06458222
NPI: 1205907805
| Primary Taxonomy | Selected Taxonomy | State | License Number |
|---|---|---|---|
| No | 111N00000X - Chiropractor | NM | DC2182 |
| Yes | 111N00000X - Chiropractor | TX | DC5807 |
| Yes | 363LF0000X - Nurse Practitioner - Family | TX | 1191402 |
| Yes | 363LF0000X - Nurse Practitioner - Family | FL | 11043890 |
| Yes | 363LF0000X - Nurse Practitioner - Family | CO | C-APN.0105610-C-NP |
| Yes | 363LF0000X - Nurse Practitioner - Family | NY | N25929 |
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card




 Again, We Welcome You.
Again, We Welcome You.
Comments are closed.