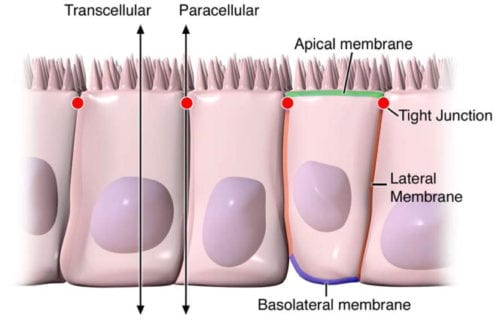
Digestive health can be attributed to the optimal function of the gastrointestinal tract. By way of instance, however, if disease affects the structure of the gut, understanding its anatomy and function, can help healthcare specialists conclude a diagnosis outcome. The intestinal epithelium is a single layer of cells found lining the intestinal lumen, which plays the role of carrying out two essential functions in the digestive system. Its first function is to act as a barrier to prevent the passage of harmful intraluminal entities, such as foreign antigens, microorganisms and their toxins. Its second function is to act as a selective filter, allowing the translocation of important dietary nutrients, electrolytes and water from the intestinal lumen to the blood stream. The intestinal epithelium distinguishes selective permeability through two main pathways: the transepithelial/transcellular and paracellular pathways, as seen on Figure 1.
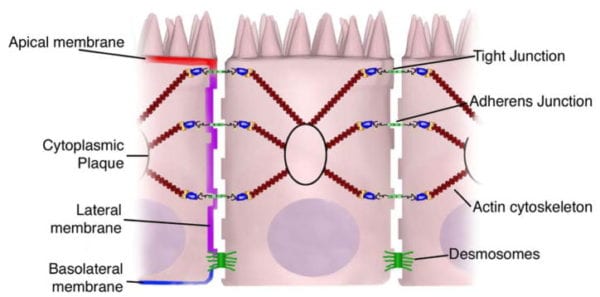
Transcellular permeability is generally related with the epithelial cells and is largely regulated by specific transporters in charge of also transferring amino acids, electrolytes, short chain fatty acids and sugars throughout the human body. Paracellular permeability is usually related to the transferring distance between epithelial cells and is greatly regulated by intercellular complexes found in the apical-lateral membrane junction and along the lateral membrane of the gastrointestinal tract, or GI tract. Interaction between the intestinal epithelial cells involves three components which can be identified at the ultrastructural level: desmosomes, adherens junctions, or AJs, and tight junctions, or TJs, as seen on Figure 2. The adhesive junctional complexes are made up of transmembrane proteins which connect adjacent cells to the actin cytoskeleton through cytoplasmic scaffolding proteins. The adherens junctions and desmosomes are believed to be more significant than the mechanical link of adjacent cells. The tight junctions, on the other hand, are the apical-most junctional complex, accountable for closing the intercellular space as well as regulating specific paracellular ionic solute transfer. The AJ and TJ complexes are also essential towards the regulation of cellular proliferation, polarization and distinction.
Table of Contents
Structural Components of Junctional Complexes
Adherens Junctions (AJs)
The adherens junctions, also known as zonula adherens, are protein complexes located along the lateral membrane which happen in points of cell to cell contact, as seen on Figure 2. They’re shaped by interactions between transmembrane proteins, intracellular adaptor proteins and the cytoskeleton. The major AJs, or adherens junctions are formed by cadherin to catenin interactions. Epithelial (E)-cadherins, or calcium-dependent adhesion molecules, are Type-I single transmembrane spanning glycoproteins that contain an intracellular C-terminus and extracellular N-terminus. The extracellular domain creates homotypical interactions with the cadherins of neighboring cells to develop this cell to cell adhesion. The intracellular domain contains a catenin-binding domain that interacts with members of the armadillo repeat superfamily, β-, γ- and p120-catenin. The catenins then connect the AJs into the cytoskeletal network through direct binding to the C-terminal domain of F-actin or indirectly through interactions with other adaptor proteins like afadin. Cadherin to catenin complexes are significant not only for connecting adjoining cells, but also for keeping cell polarity and for regulating epithelial migration and proliferation as well as the formation of additional adhesive complexes, such as desmosomes. In order to allow the link of adjoining cells, a decreased regulation of E-cadherin from the intestinal epithelium interrupts cell to cell adhesion which has been associated with affected intestinal epithelial proliferation and migration.
Nectin-afadin interactions create another significant AJ complex. Nectins, specificially nectin-1-4, are immunoglobulin-like proteins that withstand homophilic and heterophilic interactions with nectins on adjacent cells. Nectins can interact with the cytoskeleton through afadin, an F-actin binding protein, or rather preferably through interactions with other F- or α-actin binding proteins including ponsin/SH3P12, vinculin and afadin dil domain-interacting proteins.
Tight Junctions (TJs)
The tight junctions are the apical-most adhesive junctional complexes in the epithelial cells of mammals which develop a continuous belt-like ring around epithelial cells at the boundary between the apical and lateral membrane regions of the gastrointestinal tract, according to Figure 2. Tight junctions, or TJs, are powerful, multi-protein complexes which serve as a selective/semipermeable paracellular barrier, that eases the passage of ions and solutes through the intercellular space, while also preventing the translocation of luminal antigens, microorganisms and their toxins. The progression of TJ biology began in the 1960’s with the development of electron microscopy. Evaluation and analysis of epithelial cells explained a series of apparent fusions, in which the space between adjacent epithelial cells had been eliminated. These so-called “kissing points” are morphologically different from AJs and desmosomes, where adjoining cell membranes stay approximately 15 to 20nm apart. Since the first observations, TJs have been found to include four families of transmembrane proteins: occludin, claudins, junctional adhesion molecules, or JAMs, and tricellulin.
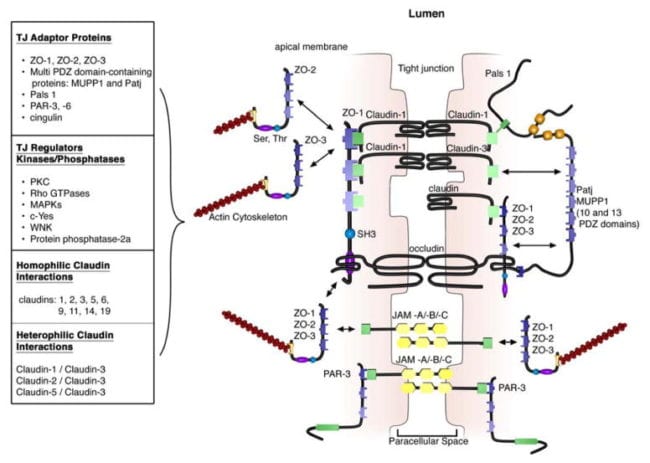
The extracellular domains of transmembrane TJ proteins in adjoining cells anastomose to shape the TJ isolate. These interactions involve those proteins found in the exact same membrane as well as those including proteins in adjacent cells. Additionally, TJ proteins may form homophilic interactions, with the exact same protein, or heterophilic interactions, between non-identical TJ proteins. Like the adherens junctions, the intracellular domains interact with different scaffolding proteins, adaptor proteins and signaling complexes to moderate cytoskeletal attachment, cell polarity, cell signaling and vesicle trafficking, as seen on Figure 3. The intracellular regions of AJs possess PDZ-binding domains, which gather and come in contact with PDZ domain containing proteins. The PDZ domain (Post synaptic density-95/Drosophila disk large/Zonula occludens-1 protein) is a common structural domain of about 80 to 90 amino acids which play the role of anchoring transmembrane proteins to the cytoskeleton. The intracellular domains may also interact with non-PDZ-binding domain including proteins like cingulin, which can interact with junctional membrane proteins, the actin cytoskeleton and signaling proteins. The complex network of intracellular protein interactions can also be known as the “cytoplasmic plaque”.
Tight Junction Formation in the Gastrointestinal Tract
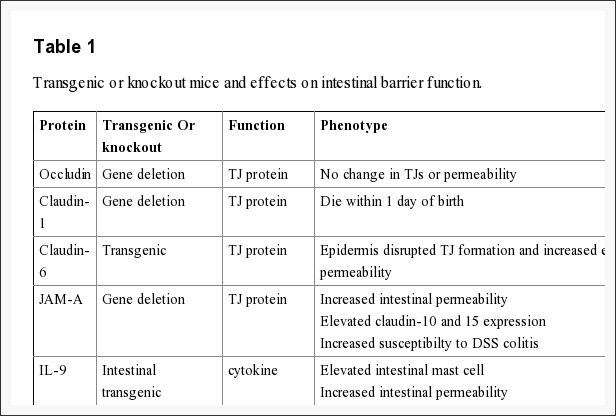
The intestinal epithelium shapes the largest and most essential barrier between our external and internal gastrointestinal tract environments. The barrier is preserved by the presence of AJs and TJs, such as cadherins, claudins, occludin and JAM proteins, which isolate groups of adjacent cells and maintains cytoskeletal anchorage, as seen on Figure 3. Expression of junctional proteins in the gut are highly regulated and dependent on both the small and/or large intestine, villus/crypt location and cell membrane specificity; apical, lateral or basolateral. The complex pattern of TJ expression from the gut is related to the particular functions of a distinct intestinal region and location. Expression of adherens junctions and tight junctions proteins can also be controlled by phosphorylation, according to Table 1. Phosphorylation can either promote TJ formation and barrier feature, or alternatively promote TJ protein redistribution and intricate destabilization.
Occludin
One of the first integral membrane proteins belonging specifically to the tight junctions to be recognized is the occludin. Occludin are predominantly found at TJs in the epithelial and endothelial cells but can also be located in astrocytes, neurons and dendritic cells. Occludin (60 to 82 kDa) is a tetraspanning integral membrane protein consisting of two extracellular loops, a short cytoplasmic N-terminus and a long cytoplasmic C-terminus. Analysis and evaluation of the function of these have demonstrated that the extracellular loops and transmembrane domains of occludin manage and maintain selective paracellular permeability. Intracellularly, the C-terminus interacts with the PDZ-domain containing protein ZO-1, which is required to connect occludin into the actin cytoskeleton, according to Figure 3.
Several occludin isoforms are characterized and believed to be the result of alternative mRNA splicing. Quite distinctly, many splice variants demonstrate altered subcellular distribution and interaction with other TJ molecules. Evaluation of these splice variants showed that the cytoplasmic C-terminal domain is funcamental for the intracellular exchange of occludin to the lateral cell membrane, which the fourth transmembrane domain name is important for targeting occludin into the TJ as well as for ZO-1 interactions.
The role of occludin is not fully outlined; nonetheless, data suggested another function for occludin from the regulation of paracellular permeability. The major allergen of the house dust mite, Der p 1, was determined to proteolyticly disrupt occludin altering this TJ complex and increasing paracellular permeability. In addition, hydrocortisone treatment of bovine retinal endothelial cells improved occludin expression two-fold and enhanced monolayer barrier properties. Though occludin is an important element of TJs, TJ formation and paracellular permeability barrier function are not dependent on occludin. Experimental investigations of occludin on mice demonstrated equivalent numbers and groups of TJs and corresponding paracellular ion passage as wild mice. Furthermore, epithelial transport and barrier function were normal in mice with occludin. Along with regulating paracellular permeability, there is evidence indicating occludin is included in cellular adhesion. Length of occludin at occludin and rat fibroblasts conferred cell to cell adhesion that has formally been interrupted by synthetic peptides associated to the first extracellular loop of occludin, underscoring the significance of the area of occludin in cell adhesion.
Evaluations indicated that occludin found along the TJ complex is regulated by phosphorylation. Several potential phosphorylation sites at tyrosine, serine, and threonine residues of occludin have been identified where the regulation of occludin phosphorylation is proposed to happen by kinases, for instance, non-receptor tyrosine kinase c-Yes and protein kinase C (PKC), and phosphatases including the serine/threonine protein phosphatase 2A, according to Figure 3. PKCη, a novel protein kinase predominantly expressed in the intestinal epithelium, was demonstrated to directly phosphorylate occludin in threonine residues (T403 and T404). Blockade of all PKCη-mediated occludin phosphorylation interrupted junctional distribution of occludin and ZO-1 and interrupted epithelial barrier function. The data suggest that occludin phosphorylation modulates occludin-ZO-1 interactions and the maintenance of intact TJ complexes and paracellular barrier function.
Claudins
Claudins are 20 to 27 kDa integral membrane proteins with four hydrophobic transmembrane domains, two extracellular loops and N- together with C-terminal cytoplasmic domains. The extracellular loops are crucial for homophilic and/or heterophilic TJ protein to protein interactions alongside the creation of ion-selective channels. The intracellular C-terminal domain is included in anchoring claudin into the cytoskeleton through connections with PDZ-binding domain names, such as ZO-1, -2 and -3, according to Figure 3. Presently, 24 distinct claudin family receptor members are identified in those who have a number of orthologues expressed in various species. They exhibit distinct cell, tissue and developmental stage-specific expression routines.
Claudin to claudin interactions between adjoining cells might be homophilic or heterophilic. Homophilic interactions have been shown for claudins 1, 2, 3, 5, 6, 9, 11, 14 and 19. On the reverse side, heterophilic interactions are more restricted and largely have been detected with claudin-3, which could interact with claudins-1, -2 and -5. Notably, there’s specificity in heterophilic trans-interactions. By way of instance, transfection of fibroblasts with claudins-1, -2 and -3 led to claudin-3 interactions with claudin-1 and -2; yet no interactions involving claudin-1 and -2 were detected. These discerning interactions are considered to describe the diversity in TJ formations and provide a molecular basis for tissue-specific heterogeneity of barrier function.
Recent study, together with claudin-deficient mice also give corroborative information supporting a role for claudins in the law of barrier function. Claudin-1 mice die within a day of birth due to significant transepidermal water loss. Furthermore, transgenic overexpression of both claudin-6 in skin disrupted tight junction formation and increased epithelial permeability. Experimental data indicates that claudins could have differential impacts on paracellular permeability. By way of instance, introduction of claudin-2 to MDCK I cells which state claudin-1 and -4 activates a decrease in transepithelial resistance, or TER; whereas transfection of claudin-3 had no effect indicating that claudin-2 markedly diminished claudin-1/claudin-4 based TJ strand regeneration. In support of the latest experimental evidence indicates that claudins can form measurements and charge-specific paracellular stations. Transfection of claudin-8 into MDCK II cells that lacks endogenous claudin-8 substantially reduced paracellular movement without impacting anion and uncharged solute movement. Experimental investigations suggest that the first extracellular loop of claudins play an essential role in deciding charge selectivity. Interchanging of the earliest or extracellular domains of claudin-4 on claudin-2 profoundly diminished the ion conductance of Na+ relative to Cl− 76. Additionally, substitution of a negatively-charged lysine into some positively charged aspartic acid (K65D) inside the loop of claudin-15 generated an increase in Na+ permeability, whereas mutation in exactly the same place of three positively charged amino acids into negatively charged aspartic acid, arginine and aspartic acid (E46K, D55R and E64K) altered the ion selectivity of claudin-15 in Na+ to Cl− channel. Pore size and density may also impact paracellular movement of non invasive and charged charged solutes.
Claudins also play an essential role in epithelial cell invasion and motility. Overexpression of claudins-3 and -4 in human ovarian epithelial cells, which lack the expression of these proteins, has been connected with enhanced epithelial cell survival and enhanced invasion and motility. Consistent with this observation, siRNA-mediated knockdown of the two claudins-3 and -4 in ovarian cancer cell lines diminished intrusion. The outcome of claudin-3 appear to get connected to altered matrix metalloprotease-2 activity, meaning claudin-induced invasion could possibly be regulated by metalloprotease proteins.
Similar to occludin, claudin localization to the TJ complex and its function are regulated by post-translational phosphorylation and through connections with PDZ-binding domains. The intracellular C-terminal domain of claudin possesses multiple regulatory sites, such as possible serine and theronine phosphorylation sites and PDZ-binding domain names. Phosphorylation of claudins-3 and -4 in prostate cancer cells is closely connected to the regulation of paracellular permeability. By way of instance, patients with pseudohypoaldosteronism type II (PHA II; or vitamin shunt syndrome) present with hyperkalemic metabolic acidosis, hypertension and dysregulated paracellular ion transport. The molecular basis is connected to some loss-of-function mutation from the serine-threonine kinases, WNK1 and WNK4, which regulate epithelial chloride cotransporters. This also contributes to an increase in the phosphorylation of both claudins-1-4 and an increase in paracellular permeability. A lot of signaling pathways are implicated in the phosphorylation of claudins like PKC, Rho GTPases, mitogen-activated protein kinases (MAPKs) and phosphatases. MAPK phosphorylation of claudin-1 is required for claudin-1-mediated barrier function. Furthermore, claudins-1, -2, -7, -8, -16 and -17 have putative PKC phosphorylation websites.
All claudins, except claudin-12, completing from the dipeptide arrangement YV, that’s been shown to interact with PDZ-binding domains comprise ZO-1, -2 and -3, multi-PDZ domain name and PALS1-associated TJ protein, according to Figure 3. Several of those scaffolding proteins contain several PDZ domains, which eases the introduction of dense localized protein complexes, also called “cytoplasmic plaques”. Also, the scaffolding proteins can interact with signaling molecules, such as heterodimeric GTP binding proteins (Rab13 and Gα12), transcriptional factors and RNA-processing variables, to connect TJ complexes to the actin-cytoskeleton and modulate aspects of adrenal polarization, differentiation and barrier function.
Junctional Adhesion Molecules (JAMs)
Junctional adhesion molecules are integral membrane proteins which belong to the immunoglobulin superfamily and have two immunoglobulin folds, the VH- and C2-type, from the extracellular domain. JAMs are expressed by multiple cell types, including epithelial, endothelial and immune cells. They’re subdivided based on the expression of Type I or II PDZ-binding themes in the intracellular C-terminus, which implies that the two types interact with exceptional scaffolding and cytoplasmic proteins. JAM-A, -B and -C (or JAM1-3) have Type II binding subjects, while the atypical JAMs, such as JAM-4, coxsackie and adenovirus receptor (CAR) and endothelial selective adhesion molecule make up Type I PDZ-binding domains. Comparable to additional TJ proteins, these JAM-PDZ interactions provide anchorage to the actin cytoskeleton, according to Figure 3.
The extracellular region of JAMs adapting to multiple ligands through homophilic and heterophilic interactions, which can be proposed to regulate the mobile functions and paracellular permeability of JAMs. Homophilic JAM-A or -B interactions govern the creation of operational TJs and cell to cell border formation, while heterophilic JAM interactions play a role in leukocyte-endothelial cell adhesion.
Recent studies demonstrate the significance of JAM-A at the formation and assembly of TJs in intestinal epithelial cells. SiRNA downregulation of JAM-A at SK-C015 epithelial cells triggered an increase in permeability. Consistent with this, JAM-A mice had increased mucosal permeability as indicated by enhanced dextran flux and decreased TER. Nonetheless, these mice also had an increase in claudin-10 and -15 expression, which is believed to shape selective pores from the TJ complex, improving paracellular permeability. Interestingly, JAM-A mice have increased susceptibility to chemical-induced colitis. Dextran sodium sulfate administration to JAM-A mice induced more acute colonic injury as compared to WT control animals. These studies imply altered intestinal permeability for a susceptibility factor to autoimmune disorder.
The above information is evidence-based. The scope of our information is limited to chiropractic and spinal injuries and conditions. To discuss the subject matter, please feel free to ask Dr. Jimenez or contact us at 915-850-0900 .
By Dr. Alex Jimenez

Additional Topics: Wellness
Overall health and wellness are essential towards maintaining the proper mental and physical balance in the body. From eating a balanced nutrition as well as exercising and participating in physical activities, to sleeping a healthy amount of time on a regular basis, following the best health and wellness tips can ultimately help maintain overall well-being. Eating plenty of fruits and vegetables can go a long way towards helping people become healthy.

TRENDING TOPIC: EXTRA EXTRA: How to Become a Healthier You!
Post Disclaimer
Professional Scope of Practice *
The information herein on "Junction Complex Structure & Function in the GI Tract" is not intended to replace a one-on-one relationship with a qualified health care professional or licensed physician and is not medical advice. We encourage you to make healthcare decisions based on your research and partnership with a qualified healthcare professional.
Blog Information & Scope Discussions
Welcome to El Paso's Premier Wellness, Personal Injury Care Clinic & Wellness Blog, where Dr. Alex Jimenez, DC, FNP-C, a Multi-State board-certified Family Practice Nurse Practitioner (FNP-BC) and Chiropractor (DC), presents insights on how our multidisciplinary team is dedicated to holistic healing and personalized care. Our practice aligns with evidence-based treatment protocols inspired by integrative medicine principles, similar to those on this site and our family practice-based chiromed.com site, and focuses on restoring health naturally for patients of all ages.
Our areas of multidisciplinary practice include Wellness & Nutrition, Chronic Pain, Personal Injury, Auto Accident Care, Work Injuries, Back Injury, Low Back Pain, Neck Pain, Migraine Headaches, Sports Injuries, Severe Sciatica, Scoliosis, Complex Herniated Discs, Fibromyalgia, Chronic Pain, Complex Injuries, Stress Management, Functional Medicine Treatments, and in-scope care protocols.
Our information scope is multidisciplinary, focusing on musculoskeletal and physical medicine, wellness, contributing etiological viscerosomatic disturbances within clinical presentations, associated somato-visceral reflex clinical dynamics, subluxation complexes, sensitive health issues, and functional medicine articles, topics, and discussions.
We provide and present clinical collaboration with specialists from various disciplines. Each specialist is governed by their professional scope of practice and their jurisdiction of licensure. We use functional health & wellness protocols to treat and support care for musculoskeletal injuries or disorders.
Our videos, posts, topics, and insights address clinical matters and issues that are directly or indirectly related to our clinical scope of practice.
Our office has made a reasonable effort to provide supportive citations and has identified relevant research studies that support our posts. We provide copies of supporting research studies upon request to regulatory boards and the public.
We understand that we cover matters that require an additional explanation of how they may assist in a particular care plan or treatment protocol; therefore, to discuss the subject matter above further, please feel free to ask Dr. Alex Jimenez, DC, APRN, FNP-BC, or contact us at 915-850-0900.
We are here to help you and your family.
Blessings
Dr. Alex Jimenez DC, MSACP, APRN, FNP-BC*, CCST, IFMCP, CFMP, ATN
email: [email protected]
Multidisciplinary Licensing & Board Certifications:
Licensed as a Doctor of Chiropractic (DC) in Texas & New Mexico*
Texas DC License #: TX5807, Verified: TX5807
New Mexico DC License #: NM-DC2182, Verified: NM-DC2182
Multi-State Advanced Practice Registered Nurse (APRN*) in Texas & Multi-States
Multi-state Compact APRN License by Endorsement (42 States)
Texas APRN License #: 1191402, Verified: 1191402 *
Florida APRN License #: 11043890, Verified: APRN11043890 *
Colorado License #: C-APN.0105610-C-NP, Verified: C-APN.0105610-C-NP
New York License #: N25929, Verified N25929
License Verification Link: Nursys License Verifier
* Prescriptive Authority Authorized
ANCC FNP-BC: Board Certified Nurse Practitioner*
Compact Status: Multi-State License: Authorized to Practice in 40 States*
Graduate with Honors: ICHS: MSN-FNP (Family Nurse Practitioner Program)
Degree Granted. Master's in Family Practice MSN Diploma (Cum Laude)
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card
Licenses and Board Certifications:
DC: Doctor of Chiropractic
APRNP: Advanced Practice Registered Nurse
FNP-BC: Family Practice Specialization (Multi-State Board Certified)
RN: Registered Nurse (Multi-State Compact License)
CFMP: Certified Functional Medicine Provider
MSN-FNP: Master of Science in Family Practice Medicine
MSACP: Master of Science in Advanced Clinical Practice
IFMCP: Institute of Functional Medicine
CCST: Certified Chiropractic Spinal Trauma
ATN: Advanced Translational Neutrogenomics
Memberships & Associations:
TCA: Texas Chiropractic Association: Member ID: 104311
AANP: American Association of Nurse Practitioners: Member ID: 2198960
ANA: American Nurse Association: Member ID: 06458222 (District TX01)
TNA: Texas Nurse Association: Member ID: 06458222
NPI: 1205907805
| Primary Taxonomy | Selected Taxonomy | State | License Number |
|---|---|---|---|
| No | 111N00000X - Chiropractor | NM | DC2182 |
| Yes | 111N00000X - Chiropractor | TX | DC5807 |
| Yes | 363LF0000X - Nurse Practitioner - Family | TX | 1191402 |
| Yes | 363LF0000X - Nurse Practitioner - Family | FL | 11043890 |
| Yes | 363LF0000X - Nurse Practitioner - Family | CO | C-APN.0105610-C-NP |
| Yes | 363LF0000X - Nurse Practitioner - Family | NY | N25929 |
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card




 Again, We Welcome You.
Again, We Welcome You.
Comments are closed.