The gastrointestinal tract, or GI tract, is an essential organ system found within humans, and other animals, which functions when we consume food or water in order to begin the digestive process to extract as well as absorb energy and nutrients to finally expel the remaining waste in stool through bowel movements. The absorption of energy and nutrients along the gastrointestinal tract provides the proper nourishment for other structures to function accordingly, however, this function may also be altered due to gastrointestinal disease, or GI disease.
Table of Contents
How can intestinal barrier dysfunction lead to gastrointestinal disease?
The intestinal epithelium is a single layer of cells which functions as one of the largest and most essential barriers against the external environment. Intestinal barrier function is needed, not only for the absorption of energy and nutrients, as well as water, but also to maintain an effective defense against intraluminal toxins, antigens and enteric flora. Chemical changes within the body and other functions can lead to intestinal barrier dysfunction and increase intestinal permeability, which may lead to a variety of issues and is generally a sign of gastrointestinal disease, or GI disease.
Cytokine-Mediated
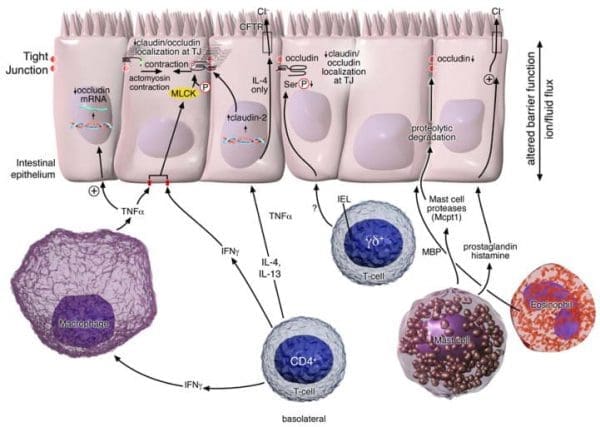
The effects of cytokine-mediated evaluations tested in vitro and in vivo animal studies demonstrated that intestinal permeability, that is the state or quality of a material or membrane which allows liquids or gasses to pass through it, can also be regulated by multiple variables, including exogenous components, relating to or developing from external factors and epithelial apoptosis, or programmed cell death, involving cytokines and immune cells, as shown on Figure 1. Immune-induced intestinal barrier dysfunction has been considered to be significant factor in the predisposition of obesity and the exacerbation of many inflammatory and autoimmune diseases, such as inflammatory bowel disease, or IBD, food allergies, autoimmune disease and diabetes. By way of instance, interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα), which are believed to be key mediators of intestinal inflammatory diseases, such as IBD, triggering intestinal epithelial barrier function. The evolution of intestinal epithelial cell monolayers (Caco2 and T84) with IFNγ and TNFα supported the reorganization of several TJ proteins: ZO-1, JAM-A, occludin, claudins-1 and -4 while it decreased epithelial barrier function. The mechanism of action of the cytokines seems to be mainly controlled through myosin light chain kinase (MLCK)-mediated phosphorylation of myosin light chain (MLC), which increases the interrupted function of the intestinal tight junctions. In support of this, inhibition of both TNFα- and IFNγ-induced MLC phosphorylation was demonstrated to restore barrier structure and function. Alternatively, TNFα and IFNγ can interrups TJs and promote intestinal permeability through dysregulation of claudin and occludin expression.
Clinical and experimental evidence-based data supports the role of Th2 cytokines in the regulation of intestinal barrier function, as seen in Figure 1. In one research study, the stimulation of colonic epithelial cells (T84 and HT-29/B6) with IL-4 or -13 triggered an increase in intestinal permeability. Quite distinctly, changes in intestinal barrier function were associated with the beginning of epithelial apoptosis and expression of the pore-forming TJ claudin-2. In vitro data suggests that the consequences of IL-4 and -13 on barrier function are primarily regulated by phosphoinositide 3-kinases. Blocking phosphoinositide 3-kinase while not hindering the activation of STAT-6 actually blocked IL-4/IL-13-induced intestinal barrier dysfunction, according to research studies. However, other studies in the testing of STAT-6 mice discovered a purpose for STAT-6, indicating instead at IL-4 and -13-mediated intestinal epithelial barrier dysfunction. Nonetheless, IL-4- and IL-13-induced changes in intestinal permeability, sugar consumption and bile secretion were lesser in STAT-6 deficient mice compared to WT mice.
The anti inflammatory cytokine IL-10 has additionally been proven to regulate intestinal barrier function. Stimulation of ileal sections from Sprague-Dawley rats with IL-10 demonstrated improved intestinal electroneutral sodium and chloride absorption and inhibited stimulated chloride secretion. In addition, treatment of T84 epithelial cell monolayers using IL-10 interrupted IFNγ-induced epithelial permeability. These results of the research study suggested that IL-10 plays a protective role in intestinal barrier function. Regardless, mice deficient in IL-10 still showed increased small intestinal permeability. Particularly in IL-10 mice, these developed chronic intestinal inflammation, which is a common symptom that manifests in individuals with Crohn’s Disease, including symptoms of weight loss, mucosal hyperplasia and chronic enterocolitis. Research study data demonstrated that increased intestinal permeability, that is the state of a membrane which allows liquids or gasses to pass through it, may predispose IL-10 mice to gastrointestinal disease. Consistent with this theory, increased permeability in IL-10 mice had been detected prior to GI disease onset.
A mechanistic research study, aiming to uncover the mechanism of intestinal barrier function, delineated IL-10-mediated intestinal permeability hypothesized the influence of the zonulin pathway and TNFα in relation to intestinal barrier dysfunction. Remarkably, however, inhibition of the zonulin receptor in IL-10 mice led to a decrease in intestinal permeability, decreased colonic TNFα secretion in vivo and interrupted the spontaneous development of colitis. These findings further support a role for zonulin, along with its potential function in the improvement of intestinal permeability during the development of gastrointestinal disease. The zonulin to zonulin receptor pathway has also been considered to regulate tight junction formation through PKC-dependent actin re-organization. Whether decreased intestinal barrier function in IL-10 mice is mainly because of a significant issue in the zonulin to zonulin receptor pathway or rather, an outcome of increased expression of cytokines, such as IFNγ and TNFα, still remains to be properly delineated in further in vitro and in vivo research studies.
Immune Cells
T-Cells
Anti-CD3-induced CD4+ T-cell activation in mice has been demonstrated to increase transcellular and paracellular intestinal permeability as well as promoting the release of pro-inflammatory cytokines, such as IFNγ and TNFα, as seen on Figure 1. By way of instance, injecting mice with TNFα provoked a breakdown in their intestinal barrier function, subsequently exacerbating symptoms of diarrhea as well as chemically affecting the PKCα-dependent inhibition of Na+/H+ exchange. T-cells, or T lymphocytes, a type of lymphocyte which plays a central roll in cell-mediated immunity, regulate transcellular permeability through the downregulation of Na+/K+-ATPase, and the interruption of Na+ absorption, Na+-glucose co-transport as well as clinical and experimental Cl− secretion. As a matter of fact, the deregulation of the paracellular permeability pathway is mediated through MLCK-dependent tight junction, or TJ, disruption.
Gamma/delta-positive intestinal intraepithelial lymphocytes (iIELγδ+), which can be closely connected with the basolateral side of intestinal epithelial cells, have also been involved in maintaining intestinal barrier function. In response to enteric parasitic infection, mice deficient in iIELγδ+ T-cells demonstrated to have abnormal claudin-3, occludin and ZO-1 localization, diminished occludin phosphorylation and abnormal epithelial tight junction formation. Also, the alterations in intestinal barrier function could particularly be associated to a subset of iIELγδ+ lymphocytes, which can be identified as T-cells expressing Vγ7+ encoded T-cell receptors. Reconstitution of mice deficient in iIELγδ+ T-cells with Vγ7+ iIELs, however, restored epithelial barrier function.
Mast Cells
Mast cells, also known as a mastocyte or a labrocyte, is a type of white blood cell that exists in most sections of the gastrointestinal tract, or GI tract. When stimulated, they release a strong collection of inflammatory mediators, such as histamine, 5-hydroxytryptamine, or 5-HT, neutral proteases (tryptases, chymases and carboxypeptidase A), prostaglandins, leukotrienes, platelet activating factors and several cytokines, including TNFα, IL-3, -4, -5, -6 and GM-CSF. Providing instances of food allergy or helminthic infestation (Nippostrongylus brasiliensis or Trichinalla spiralis), researchers have demonstrated mast cell involvement during intestinal barrier function, as seen on Figure 1. Intraluminal conflict of egg albumin-sensitized rats triggered a 15-fold growth in uptake of 51Cr-labeled EDTA when compared to rats treated with unrelated protein. The antigen-induced decreased barrier function has been connected to mast cell degranulation, a cellular process that releases antimicrobial cytotoxic or other molecules from secretory vesicles called granules, which are found inside some cells, as well as an increase in the short-circuit current, a measure of net ion transport. The significance of mast cells has been demonstrated by the lack of changes in intestinal barrier function in mast cell-deficient mice sensitized and challenged with egg albumin, which, was restored by bone marrow reconstitution. Furthermore, many mast cell mediators are proven to modulate intestinal epithelial ion transport. Pre-treatment of egg albumin-sensitized rats with histamine-H1 or 5-HT2 receptor antagonists significantly reduced oral antigen-induced short-circuit current alterations.
Clinical and experimental evaluations using models of parasitic infestation have identified a role for mast cell-derived proteases in intestinal barrier function. Murine infestation with all the enteric nematode, T. spiralis, triggered intestinal mastocytosis, occludin degradation and increased intestinal permeability. The alterations in barrier function were demonstrated to function as mast cell-dependent as the deficiency of mast cells with a neutralizing anti-c-kit antibody ablated intestinal epithelial barrier dysfunction. In addition, mice deficient in the murine mast cell protease 1, mMCP-1, were resistant to T. spiralis infestation-induced intestinal epithelial barrier dysfunction. Mast cell/MCP-1 regulation of intestinal permeability through T. Spiralis disorder were also connected to occludin degradation.
Eosinophils
Increased eosinophils and eosinophil granular proteins, including major basic proteins, eosinophil peroxidase and eosinophilic cationic protein have been regularly connected with inflammatory bowel disease, or IBD, and altered barrier function. In vitro co-culture of T84 intestinal epithelial cells together with eosinophils or eosinophil-derived major basic proteins diminished TER and improved permeability. Altered intestinal barrier function was related to the downregulation of occludin as well.
In conclusion, intestinal barrier dysfunction as well as its proper function has been connected to cytokines and immune cells. Intestinal permeability, that is the state or quality of a material or membrane which allows liquids or gasses to pass through it, can be altered due to deficiencies or interrupted function and dysfunction. The scope of our information is limited to chiropractic and spinal injuries and conditions. To discuss the subject matter, please feel free to ask Dr. Jimenez or contact us at 915-850-0900 .
By Dr. Alex Jimenez

Additional Topics: Wellness
Overall health and wellness are essential towards maintaining the proper mental and physical balance in the body. From eating a balanced nutrition as well as exercising and participating in physical activities, to sleeping a healthy amount of time on a regular basis, following the best health and wellness tips can ultimately help maintain overall well-being. Eating plenty of fruits and vegetables can go a long way towards helping people become healthy.

TRENDING TOPIC: EXTRA EXTRA: How to Become a Healthier You!
Post Disclaimer
Professional Scope of Practice *
The information herein on "Intestinal Barrier Dysfunction & TJ Formation" is not intended to replace a one-on-one relationship with a qualified health care professional or licensed physician and is not medical advice. We encourage you to make healthcare decisions based on your research and partnership with a qualified healthcare professional.
Blog Information & Scope Discussions
Welcome to El Paso's Premier Wellness, Personal Injury Care Clinic & Wellness Blog, where Dr. Alex Jimenez, DC, FNP-C, a Multi-State board-certified Family Practice Nurse Practitioner (FNP-BC) and Chiropractor (DC), presents insights on how our multidisciplinary team is dedicated to holistic healing and personalized care. Our practice aligns with evidence-based treatment protocols inspired by integrative medicine principles, similar to those on this site and our family practice-based chiromed.com site, and focuses on restoring health naturally for patients of all ages.
Our areas of multidisciplinary practice include Wellness & Nutrition, Chronic Pain, Personal Injury, Auto Accident Care, Work Injuries, Back Injury, Low Back Pain, Neck Pain, Migraine Headaches, Sports Injuries, Severe Sciatica, Scoliosis, Complex Herniated Discs, Fibromyalgia, Chronic Pain, Complex Injuries, Stress Management, Functional Medicine Treatments, and in-scope care protocols.
Our information scope is multidisciplinary, focusing on musculoskeletal and physical medicine, wellness, contributing etiological viscerosomatic disturbances within clinical presentations, associated somato-visceral reflex clinical dynamics, subluxation complexes, sensitive health issues, and functional medicine articles, topics, and discussions.
We provide and present clinical collaboration with specialists from various disciplines. Each specialist is governed by their professional scope of practice and their jurisdiction of licensure. We use functional health & wellness protocols to treat and support care for musculoskeletal injuries or disorders.
Our videos, posts, topics, and insights address clinical matters and issues that are directly or indirectly related to our clinical scope of practice.
Our office has made a reasonable effort to provide supportive citations and has identified relevant research studies that support our posts. We provide copies of supporting research studies upon request to regulatory boards and the public.
We understand that we cover matters that require an additional explanation of how they may assist in a particular care plan or treatment protocol; therefore, to discuss the subject matter above further, please feel free to ask Dr. Alex Jimenez, DC, APRN, FNP-BC, or contact us at 915-850-0900.
We are here to help you and your family.
Blessings
Dr. Alex Jimenez DC, MSACP, APRN, FNP-BC*, CCST, IFMCP, CFMP, ATN
email: [email protected]
Multidisciplinary Licensing & Board Certifications:
Licensed as a Doctor of Chiropractic (DC) in Texas & New Mexico*
Texas DC License #: TX5807, Verified: TX5807
New Mexico DC License #: NM-DC2182, Verified: NM-DC2182
Multi-State Advanced Practice Registered Nurse (APRN*) in Texas & Multi-States
Multi-state Compact APRN License by Endorsement (42 States)
Texas APRN License #: 1191402, Verified: 1191402 *
Florida APRN License #: 11043890, Verified: APRN11043890 *
Colorado License #: C-APN.0105610-C-NP, Verified: C-APN.0105610-C-NP
New York License #: N25929, Verified N25929
License Verification Link: Nursys License Verifier
* Prescriptive Authority Authorized
ANCC FNP-BC: Board Certified Nurse Practitioner*
Compact Status: Multi-State License: Authorized to Practice in 40 States*
Graduate with Honors: ICHS: MSN-FNP (Family Nurse Practitioner Program)
Degree Granted. Master's in Family Practice MSN Diploma (Cum Laude)
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card
Licenses and Board Certifications:
DC: Doctor of Chiropractic
APRNP: Advanced Practice Registered Nurse
FNP-BC: Family Practice Specialization (Multi-State Board Certified)
RN: Registered Nurse (Multi-State Compact License)
CFMP: Certified Functional Medicine Provider
MSN-FNP: Master of Science in Family Practice Medicine
MSACP: Master of Science in Advanced Clinical Practice
IFMCP: Institute of Functional Medicine
CCST: Certified Chiropractic Spinal Trauma
ATN: Advanced Translational Neutrogenomics
Memberships & Associations:
TCA: Texas Chiropractic Association: Member ID: 104311
AANP: American Association of Nurse Practitioners: Member ID: 2198960
ANA: American Nurse Association: Member ID: 06458222 (District TX01)
TNA: Texas Nurse Association: Member ID: 06458222
NPI: 1205907805
| Primary Taxonomy | Selected Taxonomy | State | License Number |
|---|---|---|---|
| No | 111N00000X - Chiropractor | NM | DC2182 |
| Yes | 111N00000X - Chiropractor | TX | DC5807 |
| Yes | 363LF0000X - Nurse Practitioner - Family | TX | 1191402 |
| Yes | 363LF0000X - Nurse Practitioner - Family | FL | 11043890 |
| Yes | 363LF0000X - Nurse Practitioner - Family | CO | C-APN.0105610-C-NP |
| Yes | 363LF0000X - Nurse Practitioner - Family | NY | N25929 |
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card





 Again, We Welcome You.
Again, We Welcome You.
Comments are closed.